Are you scouring the internet for 'write a balanced chemical equation for the combustion of methane'? All the details can be found on this website.
Table of contents
- Write a balanced chemical equation for the combustion of methane in 2021
- Ethane combustion equation
- Combustion of methane is exothermic or endothermic
- Ch4 combustion reaction
- Complete combustion of ethane
- Incomplete combustion of methane equation
- Combustion of methane equation with states
- Propane combustion equation
Write a balanced chemical equation for the combustion of methane in 2021
 This image shows write a balanced chemical equation for the combustion of methane.
This image shows write a balanced chemical equation for the combustion of methane.
Ethane combustion equation
 This picture illustrates Ethane combustion equation.
This picture illustrates Ethane combustion equation.
Combustion of methane is exothermic or endothermic
 This picture illustrates Combustion of methane is exothermic or endothermic.
This picture illustrates Combustion of methane is exothermic or endothermic.
Ch4 combustion reaction
 This image representes Ch4 combustion reaction.
This image representes Ch4 combustion reaction.
Complete combustion of ethane
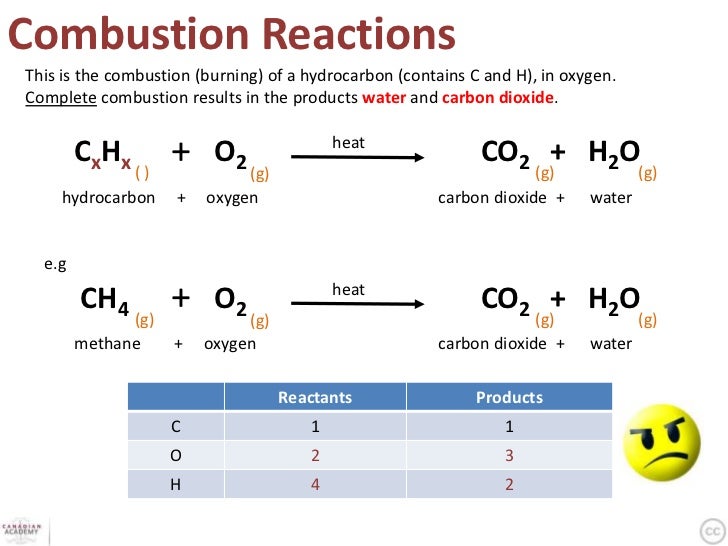 This picture shows Complete combustion of ethane.
This picture shows Complete combustion of ethane.
Incomplete combustion of methane equation
 This image demonstrates Incomplete combustion of methane equation.
This image demonstrates Incomplete combustion of methane equation.
Combustion of methane equation with states
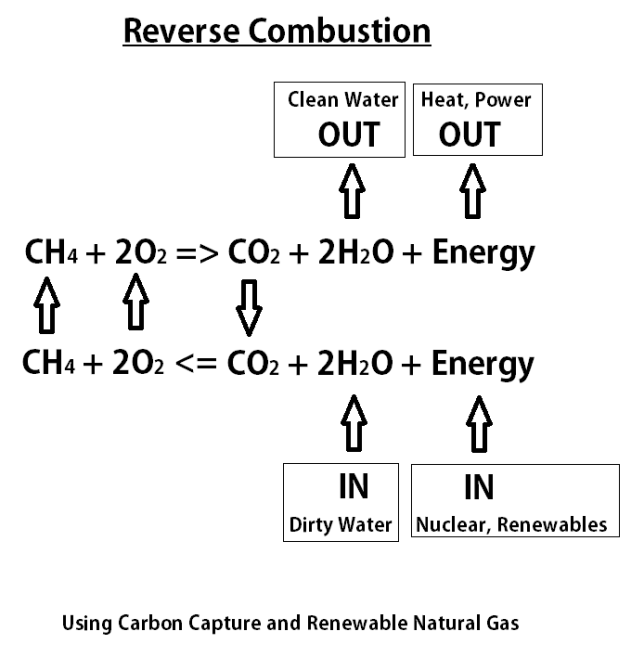 This image demonstrates Combustion of methane equation with states.
This image demonstrates Combustion of methane equation with states.
Propane combustion equation
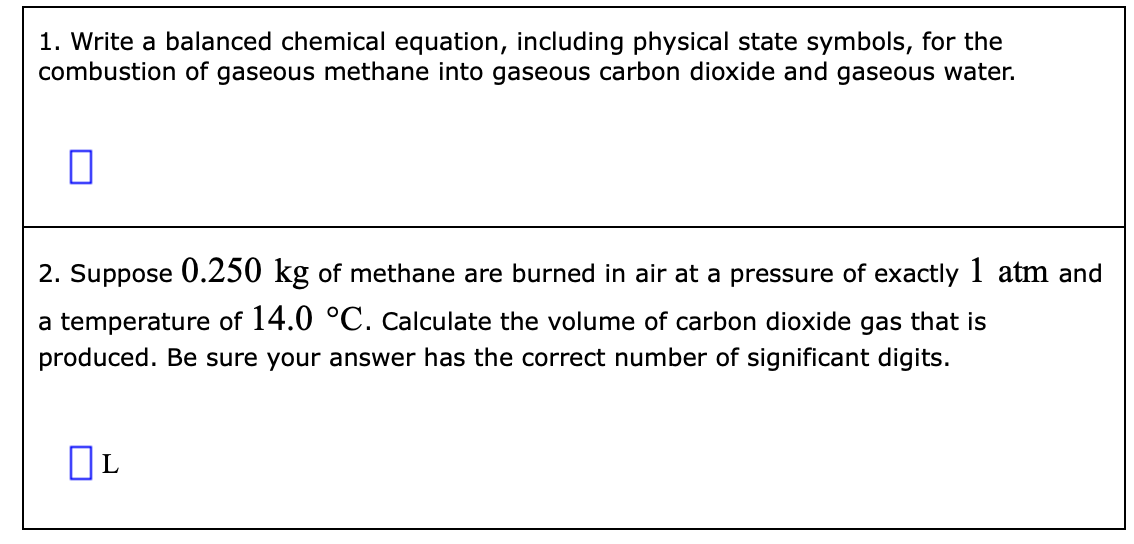 This image shows Propane combustion equation.
This image shows Propane combustion equation.
Which is the balanced equation for combustion of heptane?
C7H16 (liquid) + 11O2 (gas)--> 7CO2 (gas)+ 8H2O (gas) is the balanced equation for the combustion of heptane. What is the incomplete symbol equation for combustion of methane ch4?
What happens to carbon monoxide in CH4 combustion?
CH4 reacts with oxygen (O2) to make carbon dioxide (CO2) and water (H2O). Complete combustion does NOT give carbon monoxide or soot.
What is the balanced equation for CH4 plus O2?
Combustion. Methane is CH4 and it can undergo combustion with oxygen (O2) to form CO2 + H2O.CH4 + 2O2 ===> CO2 + 2H2O ... balanced equation What is the balanced equation for CH4 plus O2?
Which is the correct equation for methane combustion?
The balanced chemical equation for the combustion of methane is: CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (g) Which of the following statements concerning this chemical equation is/are correct? 1 . One gram of methane gas reacts with two grams of dioxygen gas, producing one gram of carbon dioxide gas and two grams of gaseous water. 2 .
Last Update: Oct 2021