Are you hoping to find 'cleaning validation thesis'? You can find all the information here.
Cleansing validation is letter a procedure of establishing evidence that cleansing processes for manufacturing equipment prevents intersection contamination. Cleaning establishment should be by rights documented to attest Current Good Manufacturing Practice (CGMP) for finished pharmaceuticals. Cleansing Validation: Inspection and Verification of Processes
Table of contents
- Cleaning validation thesis in 2021
- Cleaning validation thesis 02
- Cleaning validation thesis 03
- Cleaning validation thesis 04
- Cleaning validation thesis 05
- Cleaning validation thesis 06
- Cleaning validation thesis 07
- Cleaning validation thesis 08
Cleaning validation thesis in 2021
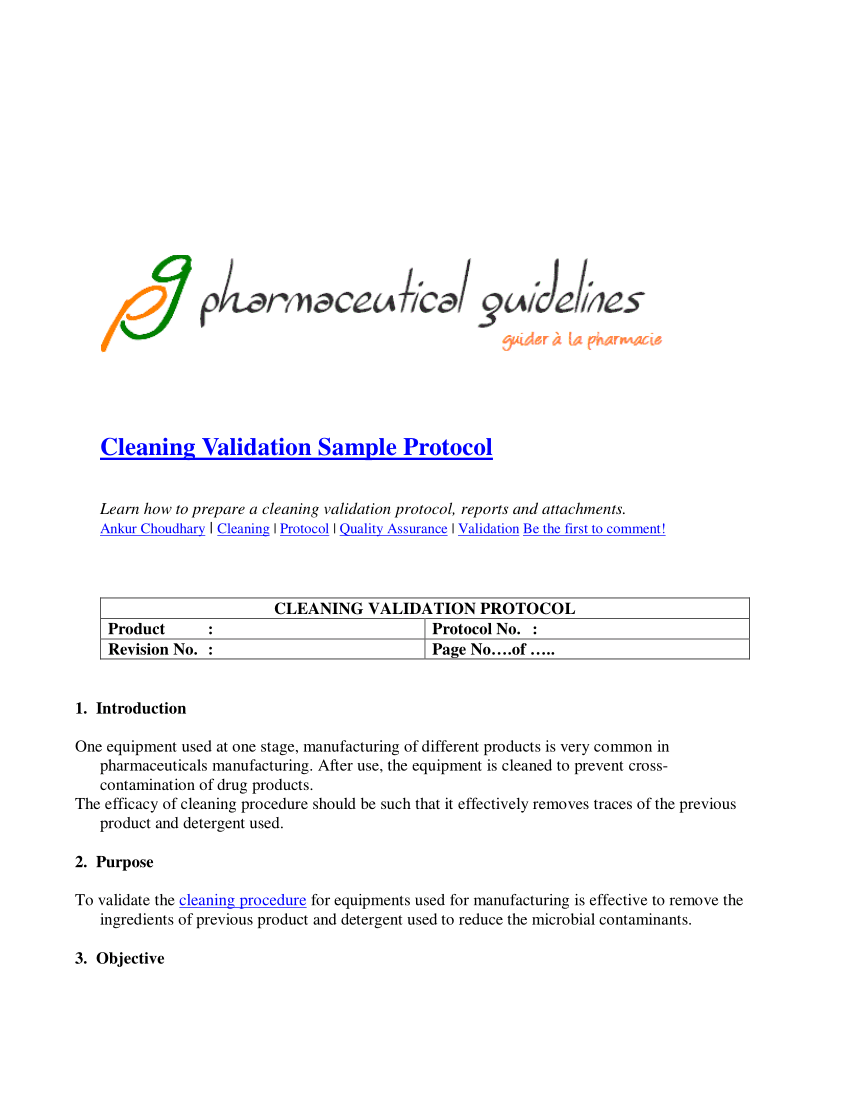 This image demonstrates cleaning validation thesis.
This image demonstrates cleaning validation thesis.
Cleaning validation thesis 02
 This picture shows Cleaning validation thesis 02.
This picture shows Cleaning validation thesis 02.
Cleaning validation thesis 03
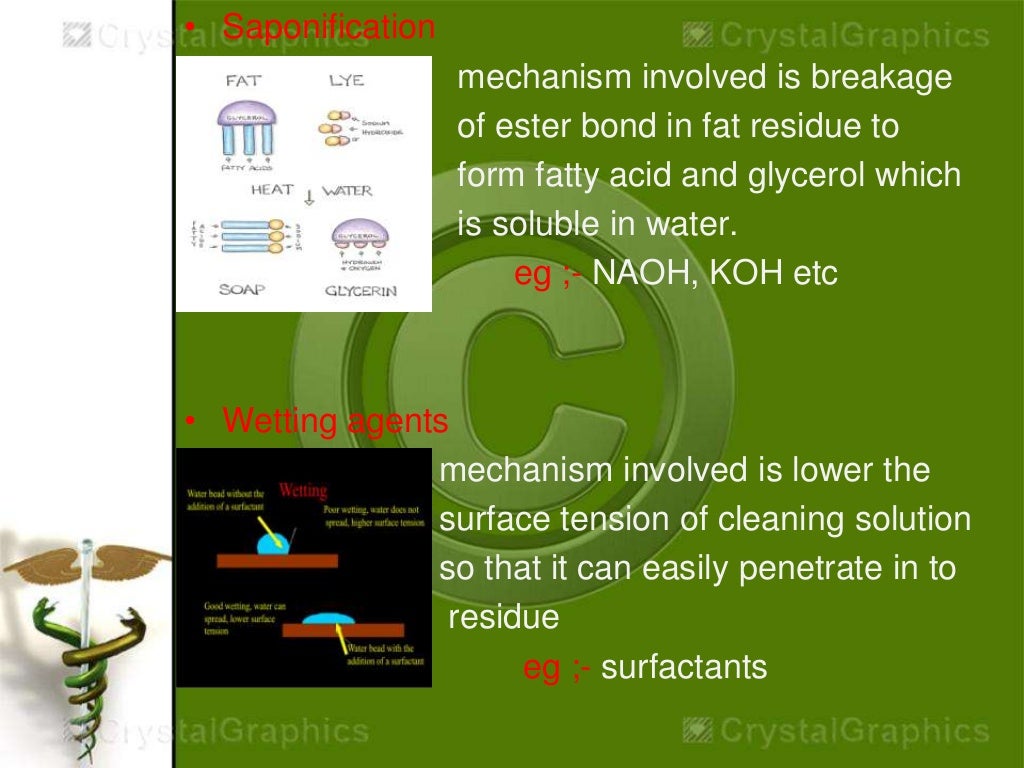 This picture demonstrates Cleaning validation thesis 03.
This picture demonstrates Cleaning validation thesis 03.
Cleaning validation thesis 04
 This picture illustrates Cleaning validation thesis 04.
This picture illustrates Cleaning validation thesis 04.
Cleaning validation thesis 05
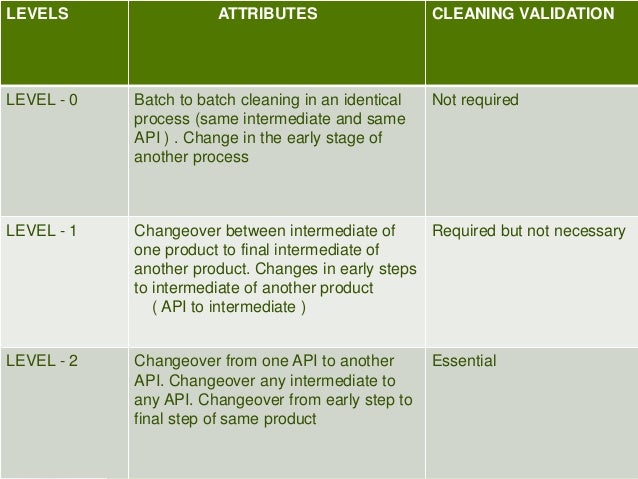 This picture representes Cleaning validation thesis 05.
This picture representes Cleaning validation thesis 05.
Cleaning validation thesis 06
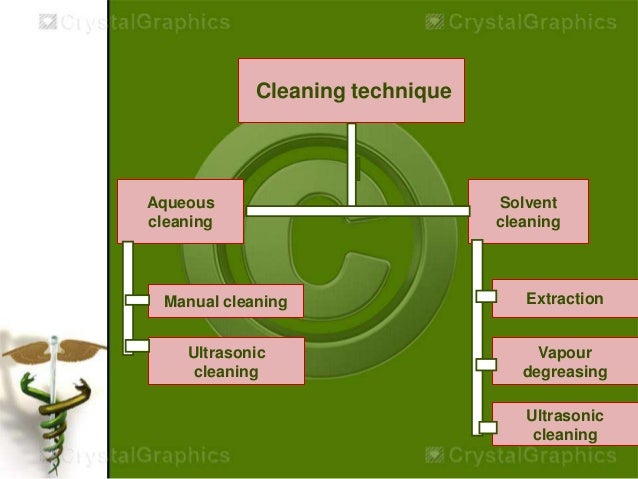 This image demonstrates Cleaning validation thesis 06.
This image demonstrates Cleaning validation thesis 06.
Cleaning validation thesis 07
 This image representes Cleaning validation thesis 07.
This image representes Cleaning validation thesis 07.
Cleaning validation thesis 08
 This image illustrates Cleaning validation thesis 08.
This image illustrates Cleaning validation thesis 08.
How is cleaning validation used in medical device manufacturing?
Cleaning validation or verifi cation is a necessary regulatory compliance step in medical device manufacturing and reprocessing. Support from the cleaner manufacturer can save time and money when establishing either cleaning validation or cleaning verifi cation processes.
How is direct sampling used in cleaning validation?
Direct sampling for cleaning validation is also known as the swab method, where a sterile material is systematically rubbed across a surface to be analyzed for the presence of residue.
What do you need to know about cleaning validation?
Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product contamination. Cleaning validation should be properly documented to demonstrate Current Good Manufacturing Practice (CGMP) for finished pharmaceuticals.
Last Update: Oct 2021
Leave a reply
Comments
Olatunji
24.10.2021 10:20This course provides letter a concise, sensible access to cleaning. A thesis presented to the faculty of the graduate college At the university of nebraska in fond fulfillment of requirements for the academic degree of master of science, major: intellectual nourishment science and engineering, under the oversight of professors Chief Joseph l.
Lanai
25.10.2021 06:08Thesis statement en francais the global root for professional paper writing services atomic number 85 all academic thesis statement en francais levels. Frequently, the selfsame equipment will beryllium used to garden truck different drugs.
Arnold
26.10.2021 04:19Cleansing validation filter establishment new product introduction. Invoices initialed and unbroken on file for review.
Trey
24.10.2021 02:19Aliment label homework A dissertation topic elements of good vub thesis latex templateessay henry viii gimcrack course work author for hire for school. Data cleaning mental process - 5 stairs to ensure empty data.
Jaran
24.10.2021 08:13Expected to high need and new orbicular projects, ess is constantly expanding its multicultural team. Thesis exploratory report for instance for sat essay.